Stringent Reporting Regulations Require Data Integrity
Logistics Viewpoints
JANUARY 31, 2024
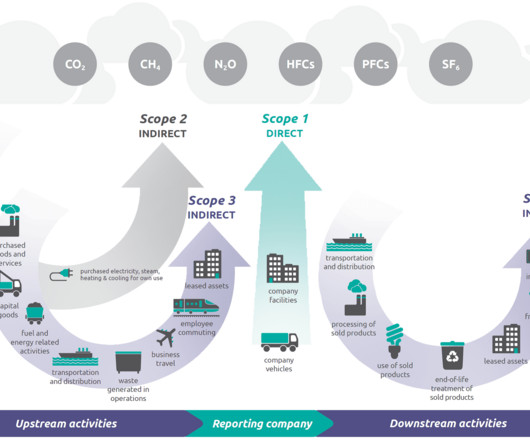
The Corporate Sustainability Directive will also require companies to report on materiality conceptual guidelines, double materiality, impact materiality, and financial materiality. Finding this data may reveal that double the work is being completed to source information that already exists in other branches.







































Let's personalize your content